优先审评凭券设想提出
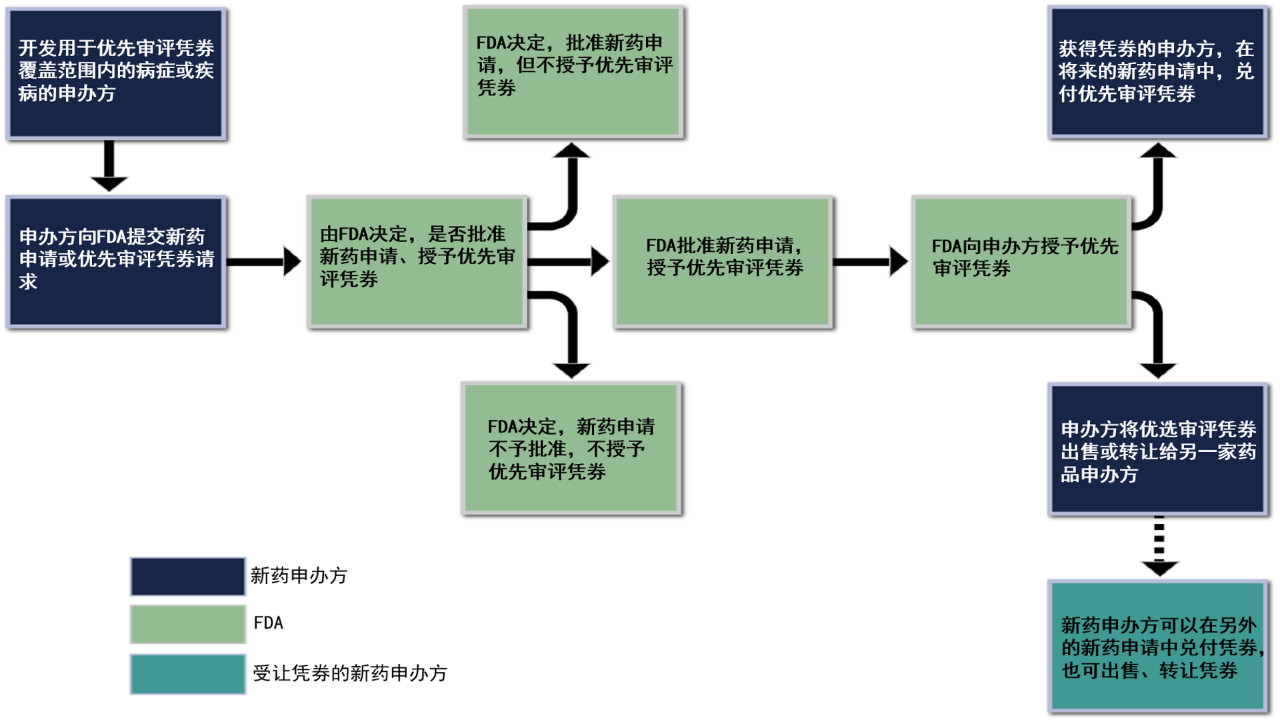
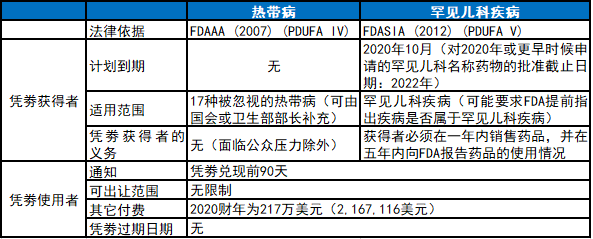
相关立法
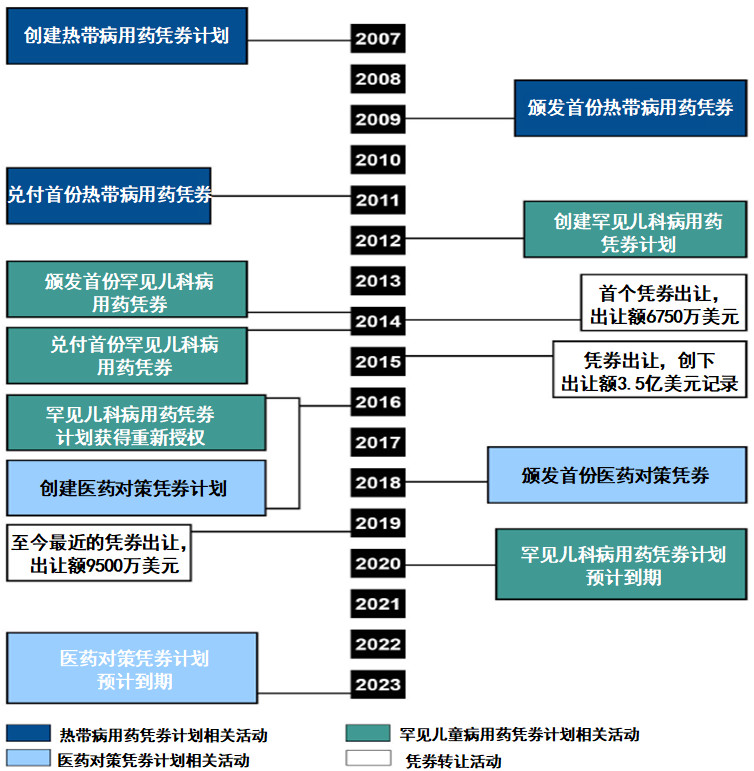
优先审评凭劵制度的影响

优先审评凭劵资质
致盲性沙眼
布鲁里溃疡
美洲锥虫病(2015年由FDA增加)
奇昆古尼亚病毒病(Chikungunya virus disease)(2018年由FDA增加)
霍乱
隐球菌脑膜炎(Cryptococcal meningitis)(2018年由FDA增加)
登革热
麦地那丝虫病
肝片吸虫病
丝状病毒(包括埃博拉病毒)(2014年通过国会立法增加)
非洲人类锥虫病(河盲症)
拉沙热(2018年由FDA增加)
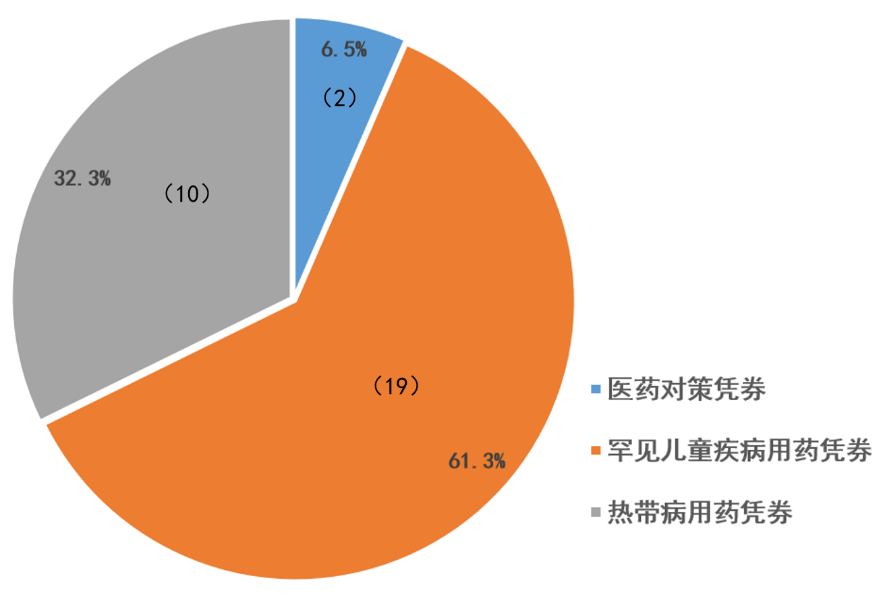
获得优先审评凭劵情况


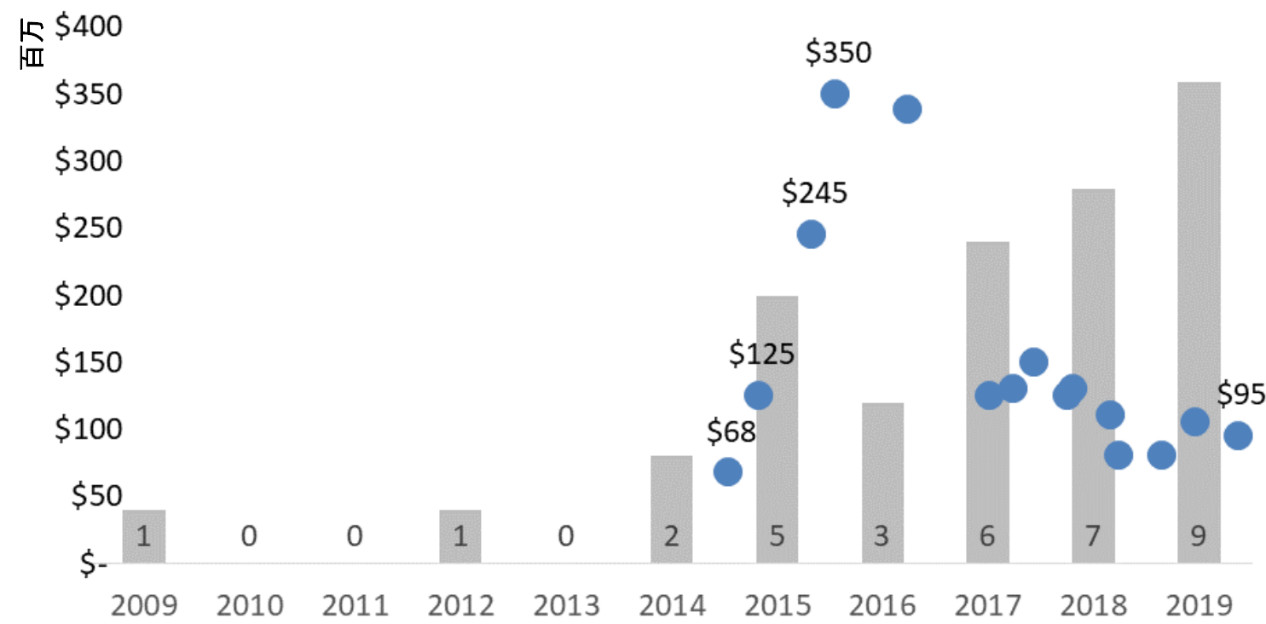
出让价格随行就市

优先审评凭劵有效期
优先审评凭劵制度的扩展
2012年《FDA安全与创新法案》(FDASIA)第908条规定了罕见儿科疾病优先审评凭劵激励计划,将凭劵计划扩展至罕见儿科疾病。获得儿科用药PRV资质的药品或生物制品必须符合下述条件:不含有以前已被FDA批准的活性成分;有资质获得优先审评(除了奖励性的优先审评外);用于治疗罕见儿科疾病;依靠研究儿科人群和用于该人群的药品剂量研究的临床数据;不寻求罕见儿科疾病用药原始申请中的成人适应症批准。
优先审评凭劵制度的局限

免责声明:本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。
参考资料:
1.David B. Ridley, Henry G. Grabowski, and Jeffrey L. Moe. Developing Drugs for Developing Countries. Health Affairs, 2006, 25(2): 313-324. doi.org/10.1377/hlthaff.25.2.313
2.GAO. DRUG DEVELOPMENT FDA’s Priority Review Voucher Programs. Jan 20, 2020. Retrieved Jan 21, 2020 from https://www.gao.gov/assets/710/704207.pdf
3. Aaron S. Kesselheim. Drug Development for Neglected Diseases: the Trouble with FDA Review Vouchers, New England Journal of Medicine, 2008, 359: 1981-1983. DOI: 10.1056/NEJMp0806684
4. Jeffrey L. Moe, David B. Ridley, and Henry G. Grabowski. FDA Review Vouchers. New England Journal of Medicine, 2009, 360: 837-838.
5. Henry G. Grabowski, David B. Ridley, and Jeff Moe. Priority Review Vouchers to Encourage Innovation for Neglected Diseases. Prescribing Cultures and Pharmaceutical Policy in the Asia-Pacific, 2009, K. Eggleston Brookings Institution Press.
6. Jason Matheny, Brad Smith, Brooke Courtney, and Michael Mair. Drug and Vaccine Development for Infectious Diseases: The Value of Priority Review Vouchers. Clinical Pharmacology & Therapeutics, 2009,85 (6): 571-572.
7. Aaron S. Kesselheim. Priority Review Vouchers: An Inefficient and Dangerous Way to Promote Neglected-Disease Drug Development. Clinical Pharmacology & Therapeutics, 2009, 85 (6): 573–575.
8. Waseem Noor. Placing Value on FDA's Priority Review Vouchers. InVivo, 2009, 27(8).
9. Jorn Sonderholm, In Defence of Priority Review Vouchers. Bioethics, 2009, 23(7): 413-420.
10. David B. Ridley and Alfonso Calles Sanchez. Introduction of European Priority Review Vouchers to Encourage Development of New Medicines for Neglected Diseases. The Lancet, 2010, 376(9744): 922-927.
11. Rianna Stefanakis, Andrew S. Robertson, Elizabeth L. Ponder, Melinda Moree. Analysis of Neglected Tropical Disease Drug and Vaccine Development Pipelines to Predict Issuance of FDA Priority Review Vouchers over the Next Decade. PLoS Neglected Tropical Diseases. 2009, 6(10): e1803.
12. Joshua S. Gans, David B. Ridley. Innovation Incentives under Transferable Fast-Track Regulatory Review. Journal of Industrial Economics,2013,61(3): 789-816.
13. David B. Ridley, Stephane A. Régnier. The Commercial Market for Priority Review Vouchers. Health Affairs, 2016,35(5): 776-783.14. David B. Ridley. Priorities for the Priority Review Voucher. The American Journal of Tropical Medicine and Hygiene, 2017. 96(1): 14–15.
15. Bavarian Nordic. Bavarian Nordic Announces the Sale of Priority Review Voucher. Dec 17, 2019. Retrieved Feb 12, 2020 from https://www.globenewswire.com/news-release/2019/12/17/1961822/0/en/Bavarian-Nordic-Announces-the-Sale-of-Priority-Review-Voucher.html
16. GW Pharmaceuticals plc. GW Pharmaceuticals plc Announces the Sale of Priority Review Voucher for $105M. Mar 18, 2019. Retrieved Feb 12, 2020 from https://www.globenewswire.com/news-release/2019/03/18/1756217/0/en/GW-Pharmaceuticals-plc-Announces-the-Sale-of-Priority-Review-Voucher-for-105M.html
17. FDA. 21st Century Cures Act: MCM-Related Cures Provisions. Oct 10, 2019. Retrieved Feb 12, 2020 from https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/21st-century-cures-act-mcm-related-cures-provisions
18. Ernst R. Berndt, Rachel Glennerster, Michael R. Kremer, et al. Advanced Market Commitments (AMCs) for Vaccines and Other Vaccines Research. Retrieved Feb 12, 2020 from https://scholar.harvard.edu/kremer/vaccine-research
19. 113th Congress. S.2917 - Adding Ebola to the FDA Priority Review Voucher Program Act. Dec 16, 2014. Retrieved Feb 12, 2020 from https://www.congress.gov/113/plaws/publ233/PLAW-113publ233.pdf
20. FDA. Office of the Commissioner. Office of the Chief Scientist. Office of Counterterrorism and Emerging Threats. Material Threat Medical Countermeasure Priority Review Vouchers. Guidance for Industry. DRAFT GUIDANCE. Jan,2018. Retrieved Feb 2, 2018 from https://www.fda.gov/media/110193/download
21. Waltz, E. FDA launches priority vouchers for neglected-disease drugs. Nat Biotechnol. 26, 1315–1316 (2008). https://doi.org/10.1038/nbt1208-1315
22. 114th Congress (2015-2016). H.R.34 - 21st Century Cures Act. Dec 13, 2016. Retrieved Dec 20, 2016 from https://www.congress.gov/bill/114th-congress/house-bill/34
23. David Ridley. Priority Review Vouchers. Retrieved Feb 12, 2020 from https://www.priorityreviewvoucher.org/
24. Businesswire. Vifor Pharma acquires Priority Review Voucher-. Feb 17, 2020. Retrieved Feb 18, 2020 from https://www.businesswire.com/news/home/20200216005042/en/Vifor